The Small-Molecule Collagen Peptide Probiotic
The Small-Molecule Collagen Peptide Probiotic is a commercial health supplement that combines probiotics with collagen peptides. Developed by National Pingtung University of Science and Technology (NPUST) and manufactured by a contracted food factory, the product is produced in compliance with NSF-GMP certification standards and has been available at the NPUST Student and Staff Consumer Cooperative since December 2021.
The product contains 12 patented probiotic strains, including Lactobacillus, Bifidobacterium, and Streptococcus thermophilus. These strains can produce short-chain fatty acids (SCFAs) and indirectly create a favorable environment for other probiotics to colonize. The fish-skin collagen peptides serve as a non-carbohydrate prebiotic, providing both nitrogen and carbon sources for intestinal probiotics. Through a specialized hydrolysis process, they are broken down into small molecules that are easily absorbed. Numerous human studies have demonstrated their anti-obesity, anti-inflammatory, antioxidant, and immunomodulatory effects.
In animal studies, probiotic supplementation attenuated weight gain and fat accumulation induced by high-fat or high-sugar diets, showing greater efficacy than the clinical anti-obesity drug Orlistat. Furthermore, it significantly improved glucose intolerance, restored hepatic and renal dysfunction (especially in male mice), and effectively reduced serum total cholesterol, triglycerides, and leptin levels. Notably, probiotic intake also alleviated anxiety induced by high-fat or high-sugar diets and enhanced spatial memory and exploratory behavior in mice. Mechanistically, the probiotic formulation exerted its benefits by improving gut microbiota diversity, including increasing Lactobacillaceae and Staphylococcaceae, while suppressing anaerobic Peptostreptococcaceae. Overall, the study confirmed that the small-molecule collagen peptide probiotic shows remarkable effects in combating obesity and its associated complications, highlighting its broad potential in promoting human health.
Subsequently, a 2024 human study recruited 46 participants and evaluated five indicators-bloating, bowel movement frequency, stool accumulation sensation, stool condition, and stool volume-before and after product use, while also assessing its impact on overall quality of life and constipation-related discomfort. Results showed that most participants experienced significant improvement in constipation severity, with moderate to severe cases benefiting the most, particularly in terms of bowel movement frequency and stool quality. Participants generally reported that the product contributed to a better quality of life.
The product contains 12 patented probiotic strains, including Lactobacillus, Bifidobacterium, and Streptococcus thermophilus. These strains can produce short-chain fatty acids (SCFAs) and indirectly create a favorable environment for other probiotics to colonize. The fish-skin collagen peptides serve as a non-carbohydrate prebiotic, providing both nitrogen and carbon sources for intestinal probiotics. Through a specialized hydrolysis process, they are broken down into small molecules that are easily absorbed. Numerous human studies have demonstrated their anti-obesity, anti-inflammatory, antioxidant, and immunomodulatory effects.
In animal studies, probiotic supplementation attenuated weight gain and fat accumulation induced by high-fat or high-sugar diets, showing greater efficacy than the clinical anti-obesity drug Orlistat. Furthermore, it significantly improved glucose intolerance, restored hepatic and renal dysfunction (especially in male mice), and effectively reduced serum total cholesterol, triglycerides, and leptin levels. Notably, probiotic intake also alleviated anxiety induced by high-fat or high-sugar diets and enhanced spatial memory and exploratory behavior in mice. Mechanistically, the probiotic formulation exerted its benefits by improving gut microbiota diversity, including increasing Lactobacillaceae and Staphylococcaceae, while suppressing anaerobic Peptostreptococcaceae. Overall, the study confirmed that the small-molecule collagen peptide probiotic shows remarkable effects in combating obesity and its associated complications, highlighting its broad potential in promoting human health.
Subsequently, a 2024 human study recruited 46 participants and evaluated five indicators-bloating, bowel movement frequency, stool accumulation sensation, stool condition, and stool volume-before and after product use, while also assessing its impact on overall quality of life and constipation-related discomfort. Results showed that most participants experienced significant improvement in constipation severity, with moderate to severe cases benefiting the most, particularly in terms of bowel movement frequency and stool quality. Participants generally reported that the product contributed to a better quality of life.
From Animal Models to Human Trials: The Health Benefits of Collagen Peptide Probiotics
Preface
Probiotics have attracted increasing attention in recent years, driven by the growing demand for health maintenance and improvement. As a key category of functional foods, probiotics demonstrate diverse and significant health benefits, and the global awareness of wellness and protection has been further amplified by the pandemic. According to statistics, the market value of Taiwan’s health supplement industry exceeded NT$170 billion between 2022 and 2023, with gastrointestinal health products achieving the highest sales. This clearly highlights the high added value and stable market growth potential of probiotic products. Looking ahead, products that integrate health and beauty benefits are expected to become the focus of consumer expectations.
The product developers, rigorously trained in scientific research, have dedicated themselves to biomedical studies and biotechnology applications. From raw material selection and formulation design to animal studies and human trials, they have progressively explored the synergistic effects of collagen peptides and probiotics. This work is not merely a series of scientific experiments or the accumulation of research data, but also a commitment to protecting and enhancing healthy living, unlocking new possibilities for innovation in functional foods.
Probiotic Research and Development Concept
Studies have clearly demonstrated that gut microbiota imbalance is closely associated with the onset of various diseases. Consuming probiotics capable of colonizing the gut helps maintain a healthy intestinal microecology. The development of effective probiotics not only relies on the precise selection and combination of species and strains but also requires consideration of their synergistic interactions with natural extracts, water-soluble dietary fibers, sugar alcohols, and other nutrients. Through the interplay of these components, targeted physiological benefits can be achieved, providing suitable options for different population groups.
Clinical data indicate that approximately 25% of the domestic population suffers from constipation, with a higher prevalence among women and the elderly, and around 30% of elementary school children are also affected. Among the wide range of probiotic products, those marketed for “digestive regularity” consistently achieve the highest sales, reflecting the urgency of this need. Moreover, consumers’ willingness to repurchase is often influenced by how quickly noticeable benefits can be experienced. On the other hand, market trends reveal continuous growth in health supplements with beauty-enhancing effects. Functional ingredients such as collagen have become highly recognized, driving the launch of innovative products that incorporate collagen to meet consumer expectations.
Therefore, probiotic development must focus on holistic formulation design, aiming to deliver synergistic benefits for both digestive regularity and beauty enhancement. This process integrates natural product chemistry with biotechnology, ensuring that both functional ingredients and final products undergo rigorous safety and efficacy verification. Supported by robust scientific data and strict quality control from raw materials to finished products, we strive to offer sustainable probiotic solutions that promote well-being and safeguard the health of all age groups.
Mechanisms of Gut Microbiota and Probiotic Development Framework
1. Select strains capable of rapidly producing large amounts of beneficial secondary metabolites
The product features a formulation of twelve probiotic strains, classified into three major groups:
(1) Lactic acid bacteria (Lactobacillus, Streptococcus)
(2) Bifidobacteria (Bifidobacterium)
(3) Auxiliary fermentative bacteria (S. thermophilus).
Each group is capable of producing beneficial secondary metabolites that support intestinal health. Lactic acid bacteria and bifidobacteria serve as the primary probiotics, while Streptococcus thermophilus functions as the “powerful support” within the probiotic formula, responsible for optimizing the microenvironment, providing resources, and protecting the primary strains. This supportive role is crucial for the establishment and stability of the entire microbial ecosystem.
The first group, the lactic acid bacteria combination, includes seven strains: L. acidophilus, L. casei, L. bulgaricus, L. plantarum, L. rhamnosus, L. paracasei, and L. helveticus. These strains consistently produce short-chain fatty acids (SCFAs) and are all capable of generating large amounts of lactic acid. Among them, L. acidophilus and L. plantarum also notably produce acetic acid, indirectly creating an ideal environment that facilitates the colonization of propionic acid- and butyric acid-producing bacteria. Under diverse carbon source conditions, L. plantarum can additionally produce propionic acid.
The second group, consisting of Bifidobacterium strains, includes four beneficial strains: B. longum, B. animalis, B. breve, and B. bifidum. These strains utilize the Bifid shunt pathway and are currently recognized as the most important representative probiotics in the human gut, capable of rapidly producing large amounts of acetic acid. In addition, this group of probiotics can generate γ-aminobutyric acid (GABA), a neuroactive compound that supports gut–brain axis health, as well as vitamins B1, B2, and folate, which help repair intestinal tissues. They also produce exopolysaccharides (EPS) and glycoside hydrolases that facilitate the breakdown of oligosaccharides. Bifidobacteria are particularly abundant and essential beneficial microbes in the infant gut, especially in breastfed infants.
Within the strain selection framework, Streptococcus thermophilus serves as a commonly used auxiliary probiotic. Although it is not primarily focused on the production of secondary metabolites and is not classified as a colonizing probiotic, it plays an important role in synbiotic synergy by promoting interactions within the gut microbiota through its own metabolism. Research has shown that S. thermophilus mainly functions by hydrolyzing lactose with β-galactosidase, providing glucose and galactose to Lactobacillus and Bifidobacterium; by rapidly fermenting lactose to lactic acid, lowering pH, and inhibiting the growth of pathogenic bacteria; and by co-fermenting with lactic acid bacteria to produce amino acids and B vitamins, thereby enhancing the overall activity of the microbiota. These findings indicate that S. thermophilus, commonly found in fermented dairy products, exerts multiple synergistic effects in supporting the probiotic ecosystem.
Acetate, propionate, and butyrate are all short-chain fatty acids (SCFAs), consisting of 2 to 5 carbon atoms, and are primarily produced by the fermentation of dietary fibers, oligosaccharides, and resistant starches by gut microbiota. They can help alleviate constipation through four main mechanisms:
(1) Promoting Intestinal Peristalsis:
• Acetate and propionate can bind to certain G protein–coupled receptors (GPCRs) located on enteric neurons and enteroendocrine cells, activating the phospholipase C (PLC) signaling pathway, which subsequently stimulates smooth muscle contraction and drives intestinal peristalsis.
• Acetate can also enhance the expression of brain-derived neurotrophic factor (BDNF) in enteric neurons, indirectly influencing the vagus nerve and the enteric nervous system to promote the release of calcitonin gene-related peptide (CGRP) and serotonin (5-HT). These two neurotransmitters act on intestinal smooth muscle and neural receptors, respectively, facilitating bowel movements. The mechanism of some clinical constipation medications is similarly based on reinforcing 5-HT–related pathways.
(2) Softening Stool and Increasing Lubrication
• Acetate can modulate ion channels in colonic epithelial cells, increasing sodium and chloride absorption and driving water reabsorption, resulting in stool with optimal water content and softness.
• Peptide YY (PYY), released in response to propionate stimulation, similarly acts on epithelial ion channels to enhance stool lubrication.
(3) Maintaining the Intestinal Barrier and Mucus Layer
• Acetate inhibits histone deacetylases (HDACs) in the cell nucleus, altering chromatin structure and promoting the expression of secreted high–molecular weight mucin genes, which thickens the intestinal mucus layer and facilitates smoother stool passage.
• Acetate also upregulates the expression of membrane proteins such as claudin-1 and occludin, which reinforce tight junctions and maintain barrier integrity.
(4) Regulating Immunity and Reducing Inflammation
• Acetate activates regulatory T cells, thereby reducing intestinal inflammation and preventing motility decline caused by inflammatory responses.
In summary, short-chain fatty acids (SCFAs) improve constipation through four synergistic mechanisms: stimulating intestinal peristalsis, maintaining stool hydration and softness, balancing the gut microbiota, and modulating the enteric nervous system. This approach is particularly effective for relieving functional constipation. Although the twelve-strain probiotic combination does not directly produce large amounts of butyrate, Bifidobacterium provides lactate and acetate as precursors, which can be converted to butyrate by commensal microbes. Lactobacillus helps by lowering pH and supplying carbon sources to support butyrate-producing bacteria. This interspecies metabolic collaboration, known as cross-feeding, is a crucial form of gut microbial co-metabolism. This probiotic consortium functions like a well-coordinated team: Lactobacillus and Bifidobacterium are the main forces, responsible for health regulation, while Streptococcus thermophilus acts as the support crew, first establishing a favorable environment, supplying nutrients, and protecting the primary strains. Ultimately, the synergistic effects of these twelve probiotics help promote intestinal health, ensure smooth digestion, and lay the foundation for overall well-being.
Category Strain Names Functions & Characteristics
Lactic Acid Bacteria L. acidophilus, L. casei, L. bulgaricus, L. plantarum, L. rhamnosus, L. paracasei, L. helveticus Produce lactic acid and acetic acid to regulate intestinal pH; some strains also generate propionic acid, supporting the colonization of butyrate- and propionate-producing bacteria
Bifidobacteria B. longum, B. animalis, B. breve, B. bifidum Rapidly produce acetic acid; generate GABA, vitamins B1/B2 and folate; produce EPS and glycosidases; the most important beneficial bacteria in infant gut microbiota
Supportive Strain S. thermophilus Breaks down lactose and produces lactic acid to inhibit harmful bacteria; supplies nutrients to primary probiotics; co-ferments with lactic acid bacteria to produce amino acids and B vitamins, enhancing overall microbiota activity
2. Safety and Comfort
Although probiotics are widely regarded as beneficial and generally free from known adverse effects, some individuals may experience gastrointestinal discomfort such as bloating or abdominal pain, and in rare cases, allergic reactions—especially in the presence of underlying health conditions. Therefore, beyond selecting clinically validated strains with low side-effect profiles, it is essential to control the dosage, avoiding excessive CFUs or over-complicated formulations. A daily intake of 1–10 billion CFUs is generally appropriate. Individual differences in gastrointestinal sensitivity must be considered to adjust dosing frequency, choose the right product, and seek professional medical advice when necessary.
3. Synergistic Effects of Auxiliary Ingredients
The primary purpose of auxiliary ingredients is to promote probiotic survival and colonization, thereby maximizing their health benefits. The most commonly added components include plant extracts, water-soluble dietary fibers, and various oligosaccharides. Water-soluble dietary fibers can absorb water and swell, increasing stool volume and stimulating intestinal motility. Oligosaccharides, which are selectively fermentable and non-digestible carbohydrates, resist gastric acid and small intestinal enzymes, allowing them to reach the colon and serve as substrates for the gut microbiota. These include fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), xylo-oligosaccharides (XOS), and inulin. Bifidobacterium and Lactobacillus strains carry specific oligosaccharide transport systems, β-galactosidase, and fructanase, enabling them to preferentially utilize oligosaccharides as an energy source, thereby promoting their growth and metabolic activity. Different strains show specificity toward oligosaccharide chain length and linkage type, requiring optimal pairings to achieve a “homologous synergy” effect. For example, GOS is more readily utilized by infant-type Bifidobacteria, while FOS stimulates the growth of adult-type Lactobacillus populations.
Oligosaccharides can also induce the expression of bacterial surface adhesion factors and alter cell surface charge, enhancing their ability to bind to intestinal mucins. This strengthens the stability and persistence of colonization. Moreover, oligosaccharide–bacteria adhesion complexes can form biofilms, providing a competitive advantage in excluding pathogenic bacteria and further stabilizing the gut microbial ecosystem.
Thus, oligosaccharides are not only indispensable as the primary carbon source in probiotic formulations but also serve as prebiotics. Through the mechanisms described above, they generate a symbiotic effect with probiotics, amplifying overall health benefits.
4. Value-Added Applications of Extracts
Collagen peptides, derived from collagen protein, are produced through specific hydrolysis techniques that break them down into smaller, more easily absorbed peptides with superior bioavailability. These small molecules can rapidly cross the intestinal barrier and enter the circulatory system, significantly improving absorption efficiency. Recent studies indicate that low-molecular-weight collagen peptides not only support skin care and weight management but also exhibit potential in modulating the gut environment and effectively alleviating constipation, thereby producing multiple physiological regulatory effects. Although research is still in its early stages, a human study involving 60 healthy adult participants randomly assigned to either an experimental group or a placebo group demonstrated that daily oral intake of fish-derived collagen peptides for eight weeks, with gut microbiota analyzed via 16S rRNA sequencing, significantly increased microbial diversity. Beneficial bacteria, including Lactobacillus and Bifidobacterium, were notably elevated, leading researchers to suggest that collagen peptides provide usable nitrogen and energy sources, acting similarly to prebiotics and promoting the proliferation of beneficial microbes.
Human studies on the effects of collagen peptides in improving skin elasticity and hydration are relatively comprehensive. One study recruited 114 healthy women aged 35 to 65, who consumed a specific collagen peptide supplement daily for eight weeks. Skin elasticity, wrinkle depth, and moisture content were measured with skin diagnostic instruments. Results showed that by week 4, skin elasticity improved significantly, especially in older participants. By week 8, wrinkle depth had decreased by 20%, and stratum corneum hydration increased, with effects persisting to week 12. This is likely because the Gly-Pro-Hyp and Pro-Hyp fragments in collagen peptides reach the dermis through the bloodstream, stimulating fibroblasts to produce more type I and type III collagen.
Another human study included 53 male participants aged 65 or older diagnosed with sarcopenia. Daily supplementation with collagen peptides combined with 12 weeks of resistance training led to significant reductions in body fat and greater improvements in muscle strength compared with the control group. The proposed mechanism involves collagen peptides stimulating the mTOR signaling pathway, enhancing muscle protein synthesis, and simultaneously increasing satiety, which supports calorie control and fat metabolism.
The relationship between collagen peptides, gut microbiota, skin health, and weight management is complex, and their integrated physiological mechanisms can be summarized as follows:
(1) Improving gut microbiota composition can reduce systemic inflammation, which helps maintain skin barrier function and regulate fat metabolism.
(2) Collagen peptides possess antioxidant, anti-inflammatory, and tissue-regenerative properties that affect the gut and skin while also influencing metabolic signaling pathways, such as AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR).
(3) Collagen peptides may act synergistically with specific probiotics, such as Lactobacillus plantarum and Bifidobacterium breve, to enhance intestinal barrier integrity and suppress fat accumulation.
(4) Collagen peptides have also been found to stimulate intestinal 5-HT secretion, which plays a key role in maintaining normal bowel movement rhythms, offering a natural approach to relieving constipation.
Collagen Peptide Probiotics and Their Fat-Reduction Effects in High-Fat Diet-Fed Mice
The animal experiment was approved by the Animal Management and Use Committee of National Pingtung University of Science and Technology. A total of 30 C57BL/6 mice (15 males and 15 females) were used in the study and were initially divided into a normal diet group (n=10) and a high-fat diet group (n=20) according to the type of feed. The normal diet group was fed standard laboratory chow with a fat content of approximately 11% of total calories, while the high-fat diet group was fed high-fat chow in which fat accounted for about 60% of total calories. The high-fat diet was given continuously for three weeks to establish a mouse obesity model. After three weeks, the high-fat diet group was randomly divided into two groups: one group continued to receive the high-fat diet with daily probiotic supplementation (n=10), and the other group received only the high-fat diet (n=10) as a control, for an additional twelve weeks. The probiotic dosage was determined in accordance with the guidelines of the U.S. Food and Drug Administration and international animal protection standards, using body surface area conversion to calculate the mouse-equivalent dose based on the recommended human intake.
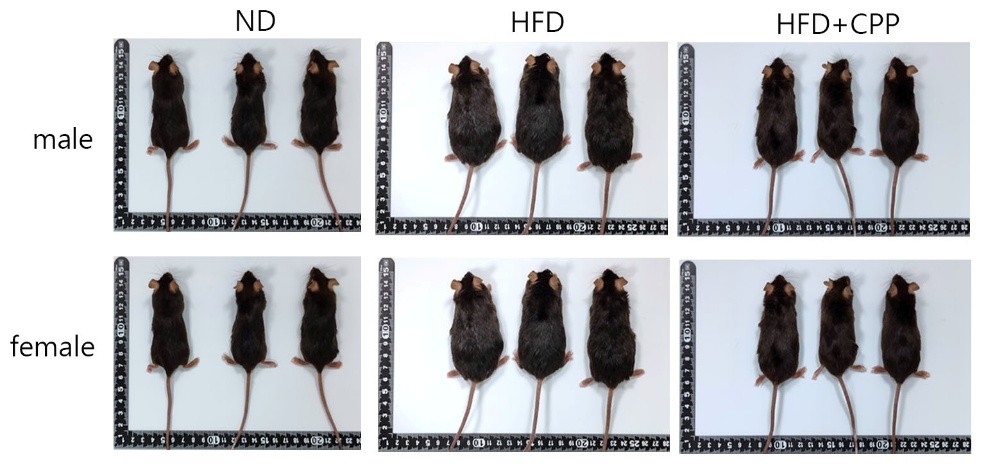
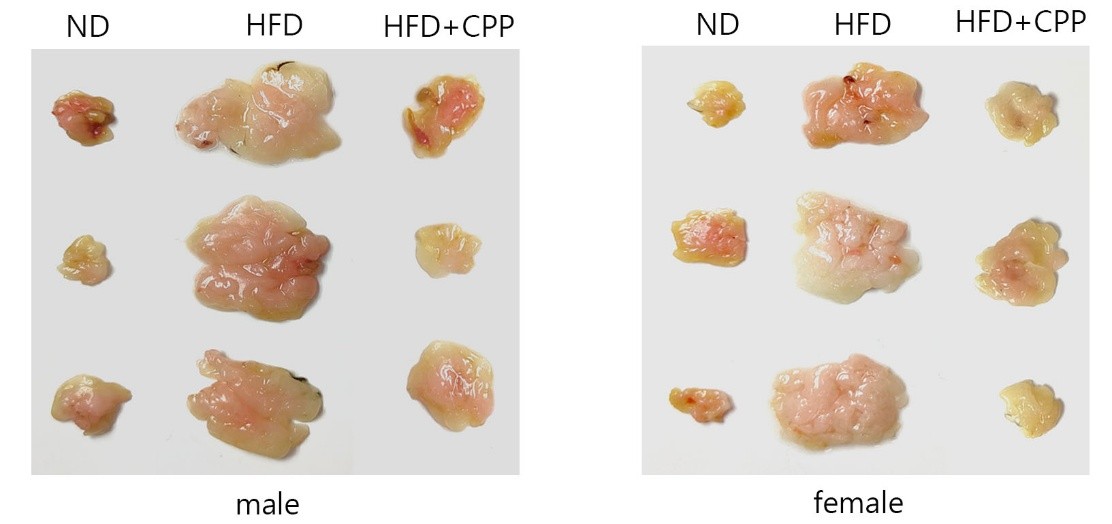
Using the body weight at week 3 as the baseline, the percentage of weight gain by week 12 was calculated. Results showed that in the high-fat diet group without probiotic supplementation, male mice exhibited a weight gain of 59.35 ± 2.22%, while female mice gained 64.64 ± 2.99%. In contrast, mice receiving probiotics demonstrated a significantly reduced weight gain, with males gaining only 33.86 ± 1.81% and females 48.76 ± 4.64%. The total experimental period lasted 15 weeks. Representative mouse body shapes are shown in Figure 1, and visceral fat content in Figure 2. By visual inspection, probiotic-supplemented mice exhibited body shapes closer to the normal diet group. Fat tissue volume in the untreated high-fat diet group was approximately 4–5 times higher than in the normal diet group, whereas probiotic supplementation markedly reduced fat volume, with a fat mass comparable to that of normal diet mice. These findings indicate that probiotics have the potential to mitigate excessive fat accumulation induced by a high-fat diet.
Figure 1 shows the body shape of the mice at the end of the 15-week experiment, with three male mice and three female mice presented for comparison.
Figure 2 shows the images of epididymal fat in male mice and peri-ovarian fat in female mice at the end of the 15-week experiment, with three individuals from each group displayed.
Most current studies on the regulation of body weight or fat metabolism by probiotics adopt a preventive model, in which probiotics are administered at the early stage of a high-fat diet or even before the initiation of high-fat feeding. However, such designs are less reflective of real clinical situations, as the actual need often arises in the intervention stage after obesity has already developed. This study employed a design different from conventional approaches: obesity was first successfully induced in mice using a high-fat diet to establish the model, and only then was probiotic supplementation introduced, simulating the clinical scenario of “already obese individuals.” Through this late-intervention model, the physiological and metabolic regulatory effects of probiotics on pre-existing obesity can be more clearly evaluated, rather than being limited to preventive outcomes.
Clinical Evidence of Collagen Peptide Probiotics in Relieving Constipation in Humans
Many studies investigating the use of probiotics to alleviate constipation have been conducted primarily in animal models. Although conversion formulas exist to estimate human-equivalent dosages from animal studies, they cannot fully determine the optimal dosage and measurable effects for human application. Therefore, based on biomedical expertise and a comprehensive integration of scientific knowledge, the author designed a composite formula combining collagen peptides with probiotics, manufactured by a certified food production facility. The clinical study protocol underwent review and approval by the Institutional Review Board (IRB), and the human trial was conducted in strict accordance with the approved protocol. The final study report was also reviewed and confirmed by the same committee.
This trial recruited participants to engage in a three-week study evaluating the effects of collagen peptide probiotics. A questionnaire was used to assess changes in constipation relief and quality of life before and after product consumption. A total of 46 participants were formally enrolled, with a balanced male-to-female ratio. The age distribution was as follows: 12 participants aged 20–29, 14 aged 30–39, 8 aged 40–49, 8 aged 50–59, and 4 aged 60 or above. Based on five self-reported indicators, participants were evaluated and scored both before and after the intervention according to the grading criteria described below.
(1) Bloating: Severe = 2 points, Mild = 1 point, None = 0 points.
(2) Bowel movement frequency: Very infrequent = 2 points, Less frequent = 1 point, Normal or more frequent = 0 points.
(3) Sensation of stool accumulation: Pronounced = 2 points, Slight = 1 point, None = 0 points.
(4) Ease of defecation: Difficult = 2 points, Slightly difficult = 1 point, Easy = 0 points.
(5) Stool volume: Less than usual = 1 point, Normal or greater = 0 points.
The total score was calculated, with 0 to 1 points defined as normal, 2 to 3 points as mild, 4 to 5 points as moderate, 6 to 7 points as moderate-to-severe, and 8 to 9 points as severe.
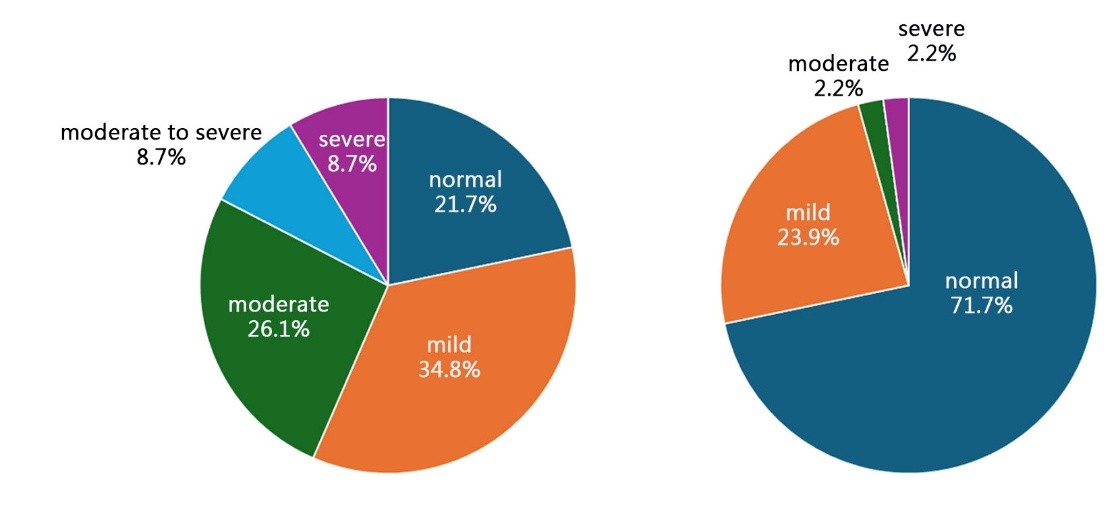
The results of the trial showed that before the intervention, among all participants, 10 were classified as normal, accounting for 21.7%; 16 as mild, accounting for 34.8%; 12 as moderate, accounting for 26.1%; 4 as moderate-to-severe, accounting for 8.7%; and 4 as severe, accounting for 8.7% (Figure 3, left). After the intervention, 33 participants were classified as normal, accounting for 71.7%; 11 as mild, accounting for 23.9%; 1 as moderate, accounting for 2.2%; 0 as moderate-to-severe, accounting for 0%; and 1 as severe, accounting for 2.2% (Figure 3, right).
Figure 3. Subjective evaluation results after three consecutive weeks of collagen peptide probiotic use by the participants, showing the changes in constipation relief and quality of life before and after the intervention, as assessed through a questionnaire survey.
From the results of this human study, it was found that one participant with severe constipation did not experience improvement after using the formula; overall, participants with pre-existing intestinal discomfort showed significant improvement, and the number and proportion of those assessed as normal increased markedly after product use, with overall quality of life improving accordingly.
Conclusion and Summary
This product features twelve carefully selected probiotic strains, designed with an emphasis on multi-genus diversity to comprehensively cover the entire intestinal microenvironment. The strains include colonizing, metabolic, and fermentative types, forming a complementary and synergistic functional network. Most of these strains are supported by clinical research and can be manufactured at scale through modern encapsulation and freeze-drying technologies, ensuring stability and viability. Data from the human study are based on participants’ real-life feedback, showing improvements from irregular or difficult bowel movements to a naturally comfortable rhythm. This reflects a journey of restoring intestinal health from the inside out, returning to an easy and comfortable daily life.
As both a scholar and product developer, it is essential to clearly convey the scientific perspective: probiotics are generally highly safe for healthy individuals and may bring multiple potential benefits. However, individual responses vary depending on constitution, dietary habits, and health status. Those with compromised immunity, existing diseases, allergies, or who are taking specific medications should consult healthcare professionals before use. Overall, under the foundation of a balanced diet and healthy lifestyle, appropriate supplementation with probiotics or health products can provide the most meaningful benefits for specific populations and circumstances.
Preface
Probiotics have attracted increasing attention in recent years, driven by the growing demand for health maintenance and improvement. As a key category of functional foods, probiotics demonstrate diverse and significant health benefits, and the global awareness of wellness and protection has been further amplified by the pandemic. According to statistics, the market value of Taiwan’s health supplement industry exceeded NT$170 billion between 2022 and 2023, with gastrointestinal health products achieving the highest sales. This clearly highlights the high added value and stable market growth potential of probiotic products. Looking ahead, products that integrate health and beauty benefits are expected to become the focus of consumer expectations.
The product developers, rigorously trained in scientific research, have dedicated themselves to biomedical studies and biotechnology applications. From raw material selection and formulation design to animal studies and human trials, they have progressively explored the synergistic effects of collagen peptides and probiotics. This work is not merely a series of scientific experiments or the accumulation of research data, but also a commitment to protecting and enhancing healthy living, unlocking new possibilities for innovation in functional foods.
Probiotic Research and Development Concept
Studies have clearly demonstrated that gut microbiota imbalance is closely associated with the onset of various diseases. Consuming probiotics capable of colonizing the gut helps maintain a healthy intestinal microecology. The development of effective probiotics not only relies on the precise selection and combination of species and strains but also requires consideration of their synergistic interactions with natural extracts, water-soluble dietary fibers, sugar alcohols, and other nutrients. Through the interplay of these components, targeted physiological benefits can be achieved, providing suitable options for different population groups.
Clinical data indicate that approximately 25% of the domestic population suffers from constipation, with a higher prevalence among women and the elderly, and around 30% of elementary school children are also affected. Among the wide range of probiotic products, those marketed for “digestive regularity” consistently achieve the highest sales, reflecting the urgency of this need. Moreover, consumers’ willingness to repurchase is often influenced by how quickly noticeable benefits can be experienced. On the other hand, market trends reveal continuous growth in health supplements with beauty-enhancing effects. Functional ingredients such as collagen have become highly recognized, driving the launch of innovative products that incorporate collagen to meet consumer expectations.
Therefore, probiotic development must focus on holistic formulation design, aiming to deliver synergistic benefits for both digestive regularity and beauty enhancement. This process integrates natural product chemistry with biotechnology, ensuring that both functional ingredients and final products undergo rigorous safety and efficacy verification. Supported by robust scientific data and strict quality control from raw materials to finished products, we strive to offer sustainable probiotic solutions that promote well-being and safeguard the health of all age groups.
Mechanisms of Gut Microbiota and Probiotic Development Framework
1. Select strains capable of rapidly producing large amounts of beneficial secondary metabolites
The product features a formulation of twelve probiotic strains, classified into three major groups:
(1) Lactic acid bacteria (Lactobacillus, Streptococcus)
(2) Bifidobacteria (Bifidobacterium)
(3) Auxiliary fermentative bacteria (S. thermophilus).
Each group is capable of producing beneficial secondary metabolites that support intestinal health. Lactic acid bacteria and bifidobacteria serve as the primary probiotics, while Streptococcus thermophilus functions as the “powerful support” within the probiotic formula, responsible for optimizing the microenvironment, providing resources, and protecting the primary strains. This supportive role is crucial for the establishment and stability of the entire microbial ecosystem.
The first group, the lactic acid bacteria combination, includes seven strains: L. acidophilus, L. casei, L. bulgaricus, L. plantarum, L. rhamnosus, L. paracasei, and L. helveticus. These strains consistently produce short-chain fatty acids (SCFAs) and are all capable of generating large amounts of lactic acid. Among them, L. acidophilus and L. plantarum also notably produce acetic acid, indirectly creating an ideal environment that facilitates the colonization of propionic acid- and butyric acid-producing bacteria. Under diverse carbon source conditions, L. plantarum can additionally produce propionic acid.
The second group, consisting of Bifidobacterium strains, includes four beneficial strains: B. longum, B. animalis, B. breve, and B. bifidum. These strains utilize the Bifid shunt pathway and are currently recognized as the most important representative probiotics in the human gut, capable of rapidly producing large amounts of acetic acid. In addition, this group of probiotics can generate γ-aminobutyric acid (GABA), a neuroactive compound that supports gut–brain axis health, as well as vitamins B1, B2, and folate, which help repair intestinal tissues. They also produce exopolysaccharides (EPS) and glycoside hydrolases that facilitate the breakdown of oligosaccharides. Bifidobacteria are particularly abundant and essential beneficial microbes in the infant gut, especially in breastfed infants.
Within the strain selection framework, Streptococcus thermophilus serves as a commonly used auxiliary probiotic. Although it is not primarily focused on the production of secondary metabolites and is not classified as a colonizing probiotic, it plays an important role in synbiotic synergy by promoting interactions within the gut microbiota through its own metabolism. Research has shown that S. thermophilus mainly functions by hydrolyzing lactose with β-galactosidase, providing glucose and galactose to Lactobacillus and Bifidobacterium; by rapidly fermenting lactose to lactic acid, lowering pH, and inhibiting the growth of pathogenic bacteria; and by co-fermenting with lactic acid bacteria to produce amino acids and B vitamins, thereby enhancing the overall activity of the microbiota. These findings indicate that S. thermophilus, commonly found in fermented dairy products, exerts multiple synergistic effects in supporting the probiotic ecosystem.
Acetate, propionate, and butyrate are all short-chain fatty acids (SCFAs), consisting of 2 to 5 carbon atoms, and are primarily produced by the fermentation of dietary fibers, oligosaccharides, and resistant starches by gut microbiota. They can help alleviate constipation through four main mechanisms:
(1) Promoting Intestinal Peristalsis:
• Acetate and propionate can bind to certain G protein–coupled receptors (GPCRs) located on enteric neurons and enteroendocrine cells, activating the phospholipase C (PLC) signaling pathway, which subsequently stimulates smooth muscle contraction and drives intestinal peristalsis.
• Acetate can also enhance the expression of brain-derived neurotrophic factor (BDNF) in enteric neurons, indirectly influencing the vagus nerve and the enteric nervous system to promote the release of calcitonin gene-related peptide (CGRP) and serotonin (5-HT). These two neurotransmitters act on intestinal smooth muscle and neural receptors, respectively, facilitating bowel movements. The mechanism of some clinical constipation medications is similarly based on reinforcing 5-HT–related pathways.
(2) Softening Stool and Increasing Lubrication
• Acetate can modulate ion channels in colonic epithelial cells, increasing sodium and chloride absorption and driving water reabsorption, resulting in stool with optimal water content and softness.
• Peptide YY (PYY), released in response to propionate stimulation, similarly acts on epithelial ion channels to enhance stool lubrication.
(3) Maintaining the Intestinal Barrier and Mucus Layer
• Acetate inhibits histone deacetylases (HDACs) in the cell nucleus, altering chromatin structure and promoting the expression of secreted high–molecular weight mucin genes, which thickens the intestinal mucus layer and facilitates smoother stool passage.
• Acetate also upregulates the expression of membrane proteins such as claudin-1 and occludin, which reinforce tight junctions and maintain barrier integrity.
(4) Regulating Immunity and Reducing Inflammation
• Acetate activates regulatory T cells, thereby reducing intestinal inflammation and preventing motility decline caused by inflammatory responses.
In summary, short-chain fatty acids (SCFAs) improve constipation through four synergistic mechanisms: stimulating intestinal peristalsis, maintaining stool hydration and softness, balancing the gut microbiota, and modulating the enteric nervous system. This approach is particularly effective for relieving functional constipation. Although the twelve-strain probiotic combination does not directly produce large amounts of butyrate, Bifidobacterium provides lactate and acetate as precursors, which can be converted to butyrate by commensal microbes. Lactobacillus helps by lowering pH and supplying carbon sources to support butyrate-producing bacteria. This interspecies metabolic collaboration, known as cross-feeding, is a crucial form of gut microbial co-metabolism. This probiotic consortium functions like a well-coordinated team: Lactobacillus and Bifidobacterium are the main forces, responsible for health regulation, while Streptococcus thermophilus acts as the support crew, first establishing a favorable environment, supplying nutrients, and protecting the primary strains. Ultimately, the synergistic effects of these twelve probiotics help promote intestinal health, ensure smooth digestion, and lay the foundation for overall well-being.
Category Strain Names Functions & Characteristics
Lactic Acid Bacteria L. acidophilus, L. casei, L. bulgaricus, L. plantarum, L. rhamnosus, L. paracasei, L. helveticus Produce lactic acid and acetic acid to regulate intestinal pH; some strains also generate propionic acid, supporting the colonization of butyrate- and propionate-producing bacteria
Bifidobacteria B. longum, B. animalis, B. breve, B. bifidum Rapidly produce acetic acid; generate GABA, vitamins B1/B2 and folate; produce EPS and glycosidases; the most important beneficial bacteria in infant gut microbiota
Supportive Strain S. thermophilus Breaks down lactose and produces lactic acid to inhibit harmful bacteria; supplies nutrients to primary probiotics; co-ferments with lactic acid bacteria to produce amino acids and B vitamins, enhancing overall microbiota activity
2. Safety and Comfort
Although probiotics are widely regarded as beneficial and generally free from known adverse effects, some individuals may experience gastrointestinal discomfort such as bloating or abdominal pain, and in rare cases, allergic reactions—especially in the presence of underlying health conditions. Therefore, beyond selecting clinically validated strains with low side-effect profiles, it is essential to control the dosage, avoiding excessive CFUs or over-complicated formulations. A daily intake of 1–10 billion CFUs is generally appropriate. Individual differences in gastrointestinal sensitivity must be considered to adjust dosing frequency, choose the right product, and seek professional medical advice when necessary.
3. Synergistic Effects of Auxiliary Ingredients
The primary purpose of auxiliary ingredients is to promote probiotic survival and colonization, thereby maximizing their health benefits. The most commonly added components include plant extracts, water-soluble dietary fibers, and various oligosaccharides. Water-soluble dietary fibers can absorb water and swell, increasing stool volume and stimulating intestinal motility. Oligosaccharides, which are selectively fermentable and non-digestible carbohydrates, resist gastric acid and small intestinal enzymes, allowing them to reach the colon and serve as substrates for the gut microbiota. These include fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), xylo-oligosaccharides (XOS), and inulin. Bifidobacterium and Lactobacillus strains carry specific oligosaccharide transport systems, β-galactosidase, and fructanase, enabling them to preferentially utilize oligosaccharides as an energy source, thereby promoting their growth and metabolic activity. Different strains show specificity toward oligosaccharide chain length and linkage type, requiring optimal pairings to achieve a “homologous synergy” effect. For example, GOS is more readily utilized by infant-type Bifidobacteria, while FOS stimulates the growth of adult-type Lactobacillus populations.
Oligosaccharides can also induce the expression of bacterial surface adhesion factors and alter cell surface charge, enhancing their ability to bind to intestinal mucins. This strengthens the stability and persistence of colonization. Moreover, oligosaccharide–bacteria adhesion complexes can form biofilms, providing a competitive advantage in excluding pathogenic bacteria and further stabilizing the gut microbial ecosystem.
Thus, oligosaccharides are not only indispensable as the primary carbon source in probiotic formulations but also serve as prebiotics. Through the mechanisms described above, they generate a symbiotic effect with probiotics, amplifying overall health benefits.
4. Value-Added Applications of Extracts
Collagen peptides, derived from collagen protein, are produced through specific hydrolysis techniques that break them down into smaller, more easily absorbed peptides with superior bioavailability. These small molecules can rapidly cross the intestinal barrier and enter the circulatory system, significantly improving absorption efficiency. Recent studies indicate that low-molecular-weight collagen peptides not only support skin care and weight management but also exhibit potential in modulating the gut environment and effectively alleviating constipation, thereby producing multiple physiological regulatory effects. Although research is still in its early stages, a human study involving 60 healthy adult participants randomly assigned to either an experimental group or a placebo group demonstrated that daily oral intake of fish-derived collagen peptides for eight weeks, with gut microbiota analyzed via 16S rRNA sequencing, significantly increased microbial diversity. Beneficial bacteria, including Lactobacillus and Bifidobacterium, were notably elevated, leading researchers to suggest that collagen peptides provide usable nitrogen and energy sources, acting similarly to prebiotics and promoting the proliferation of beneficial microbes.
Human studies on the effects of collagen peptides in improving skin elasticity and hydration are relatively comprehensive. One study recruited 114 healthy women aged 35 to 65, who consumed a specific collagen peptide supplement daily for eight weeks. Skin elasticity, wrinkle depth, and moisture content were measured with skin diagnostic instruments. Results showed that by week 4, skin elasticity improved significantly, especially in older participants. By week 8, wrinkle depth had decreased by 20%, and stratum corneum hydration increased, with effects persisting to week 12. This is likely because the Gly-Pro-Hyp and Pro-Hyp fragments in collagen peptides reach the dermis through the bloodstream, stimulating fibroblasts to produce more type I and type III collagen.
Another human study included 53 male participants aged 65 or older diagnosed with sarcopenia. Daily supplementation with collagen peptides combined with 12 weeks of resistance training led to significant reductions in body fat and greater improvements in muscle strength compared with the control group. The proposed mechanism involves collagen peptides stimulating the mTOR signaling pathway, enhancing muscle protein synthesis, and simultaneously increasing satiety, which supports calorie control and fat metabolism.
The relationship between collagen peptides, gut microbiota, skin health, and weight management is complex, and their integrated physiological mechanisms can be summarized as follows:
(1) Improving gut microbiota composition can reduce systemic inflammation, which helps maintain skin barrier function and regulate fat metabolism.
(2) Collagen peptides possess antioxidant, anti-inflammatory, and tissue-regenerative properties that affect the gut and skin while also influencing metabolic signaling pathways, such as AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR).
(3) Collagen peptides may act synergistically with specific probiotics, such as Lactobacillus plantarum and Bifidobacterium breve, to enhance intestinal barrier integrity and suppress fat accumulation.
(4) Collagen peptides have also been found to stimulate intestinal 5-HT secretion, which plays a key role in maintaining normal bowel movement rhythms, offering a natural approach to relieving constipation.
Collagen Peptide Probiotics and Their Fat-Reduction Effects in High-Fat Diet-Fed Mice
The animal experiment was approved by the Animal Management and Use Committee of National Pingtung University of Science and Technology. A total of 30 C57BL/6 mice (15 males and 15 females) were used in the study and were initially divided into a normal diet group (n=10) and a high-fat diet group (n=20) according to the type of feed. The normal diet group was fed standard laboratory chow with a fat content of approximately 11% of total calories, while the high-fat diet group was fed high-fat chow in which fat accounted for about 60% of total calories. The high-fat diet was given continuously for three weeks to establish a mouse obesity model. After three weeks, the high-fat diet group was randomly divided into two groups: one group continued to receive the high-fat diet with daily probiotic supplementation (n=10), and the other group received only the high-fat diet (n=10) as a control, for an additional twelve weeks. The probiotic dosage was determined in accordance with the guidelines of the U.S. Food and Drug Administration and international animal protection standards, using body surface area conversion to calculate the mouse-equivalent dose based on the recommended human intake.
Using the body weight at week 3 as the baseline, the percentage of weight gain by week 12 was calculated. Results showed that in the high-fat diet group without probiotic supplementation, male mice exhibited a weight gain of 59.35 ± 2.22%, while female mice gained 64.64 ± 2.99%. In contrast, mice receiving probiotics demonstrated a significantly reduced weight gain, with males gaining only 33.86 ± 1.81% and females 48.76 ± 4.64%. The total experimental period lasted 15 weeks. Representative mouse body shapes are shown in Figure 1, and visceral fat content in Figure 2. By visual inspection, probiotic-supplemented mice exhibited body shapes closer to the normal diet group. Fat tissue volume in the untreated high-fat diet group was approximately 4–5 times higher than in the normal diet group, whereas probiotic supplementation markedly reduced fat volume, with a fat mass comparable to that of normal diet mice. These findings indicate that probiotics have the potential to mitigate excessive fat accumulation induced by a high-fat diet.
Figure 1 shows the body shape of the mice at the end of the 15-week experiment, with three male mice and three female mice presented for comparison.
Figure 2 shows the images of epididymal fat in male mice and peri-ovarian fat in female mice at the end of the 15-week experiment, with three individuals from each group displayed.
Most current studies on the regulation of body weight or fat metabolism by probiotics adopt a preventive model, in which probiotics are administered at the early stage of a high-fat diet or even before the initiation of high-fat feeding. However, such designs are less reflective of real clinical situations, as the actual need often arises in the intervention stage after obesity has already developed. This study employed a design different from conventional approaches: obesity was first successfully induced in mice using a high-fat diet to establish the model, and only then was probiotic supplementation introduced, simulating the clinical scenario of “already obese individuals.” Through this late-intervention model, the physiological and metabolic regulatory effects of probiotics on pre-existing obesity can be more clearly evaluated, rather than being limited to preventive outcomes.
Clinical Evidence of Collagen Peptide Probiotics in Relieving Constipation in Humans
Many studies investigating the use of probiotics to alleviate constipation have been conducted primarily in animal models. Although conversion formulas exist to estimate human-equivalent dosages from animal studies, they cannot fully determine the optimal dosage and measurable effects for human application. Therefore, based on biomedical expertise and a comprehensive integration of scientific knowledge, the author designed a composite formula combining collagen peptides with probiotics, manufactured by a certified food production facility. The clinical study protocol underwent review and approval by the Institutional Review Board (IRB), and the human trial was conducted in strict accordance with the approved protocol. The final study report was also reviewed and confirmed by the same committee.
This trial recruited participants to engage in a three-week study evaluating the effects of collagen peptide probiotics. A questionnaire was used to assess changes in constipation relief and quality of life before and after product consumption. A total of 46 participants were formally enrolled, with a balanced male-to-female ratio. The age distribution was as follows: 12 participants aged 20–29, 14 aged 30–39, 8 aged 40–49, 8 aged 50–59, and 4 aged 60 or above. Based on five self-reported indicators, participants were evaluated and scored both before and after the intervention according to the grading criteria described below.
(1) Bloating: Severe = 2 points, Mild = 1 point, None = 0 points.
(2) Bowel movement frequency: Very infrequent = 2 points, Less frequent = 1 point, Normal or more frequent = 0 points.
(3) Sensation of stool accumulation: Pronounced = 2 points, Slight = 1 point, None = 0 points.
(4) Ease of defecation: Difficult = 2 points, Slightly difficult = 1 point, Easy = 0 points.
(5) Stool volume: Less than usual = 1 point, Normal or greater = 0 points.
The total score was calculated, with 0 to 1 points defined as normal, 2 to 3 points as mild, 4 to 5 points as moderate, 6 to 7 points as moderate-to-severe, and 8 to 9 points as severe.
The results of the trial showed that before the intervention, among all participants, 10 were classified as normal, accounting for 21.7%; 16 as mild, accounting for 34.8%; 12 as moderate, accounting for 26.1%; 4 as moderate-to-severe, accounting for 8.7%; and 4 as severe, accounting for 8.7% (Figure 3, left). After the intervention, 33 participants were classified as normal, accounting for 71.7%; 11 as mild, accounting for 23.9%; 1 as moderate, accounting for 2.2%; 0 as moderate-to-severe, accounting for 0%; and 1 as severe, accounting for 2.2% (Figure 3, right).
Figure 3. Subjective evaluation results after three consecutive weeks of collagen peptide probiotic use by the participants, showing the changes in constipation relief and quality of life before and after the intervention, as assessed through a questionnaire survey.
From the results of this human study, it was found that one participant with severe constipation did not experience improvement after using the formula; overall, participants with pre-existing intestinal discomfort showed significant improvement, and the number and proportion of those assessed as normal increased markedly after product use, with overall quality of life improving accordingly.
Conclusion and Summary
This product features twelve carefully selected probiotic strains, designed with an emphasis on multi-genus diversity to comprehensively cover the entire intestinal microenvironment. The strains include colonizing, metabolic, and fermentative types, forming a complementary and synergistic functional network. Most of these strains are supported by clinical research and can be manufactured at scale through modern encapsulation and freeze-drying technologies, ensuring stability and viability. Data from the human study are based on participants’ real-life feedback, showing improvements from irregular or difficult bowel movements to a naturally comfortable rhythm. This reflects a journey of restoring intestinal health from the inside out, returning to an easy and comfortable daily life.
As both a scholar and product developer, it is essential to clearly convey the scientific perspective: probiotics are generally highly safe for healthy individuals and may bring multiple potential benefits. However, individual responses vary depending on constitution, dietary habits, and health status. Those with compromised immunity, existing diseases, allergies, or who are taking specific medications should consult healthcare professionals before use. Overall, under the foundation of a balanced diet and healthy lifestyle, appropriate supplementation with probiotics or health products can provide the most meaningful benefits for specific populations and circumstances.